Deep Transcranial Magnetic Stimulation (Deep TMS)
Available only in St-Hubert for now.
Deep TMS at Neuroperforma – Cutting-edge technology to transform your well-being
At Neuroperforma, our mission is to make the best advancements in neurotechnology accessible, providing our clients with effective, proven solutions to improve their well-being and transform their quality of life.
Among these solutions, we are proud to be one of the few private clinics in Quebec to offer Deep Transcranial Magnetic Stimulation (Deep TMS), an innovative approach recognized worldwide.
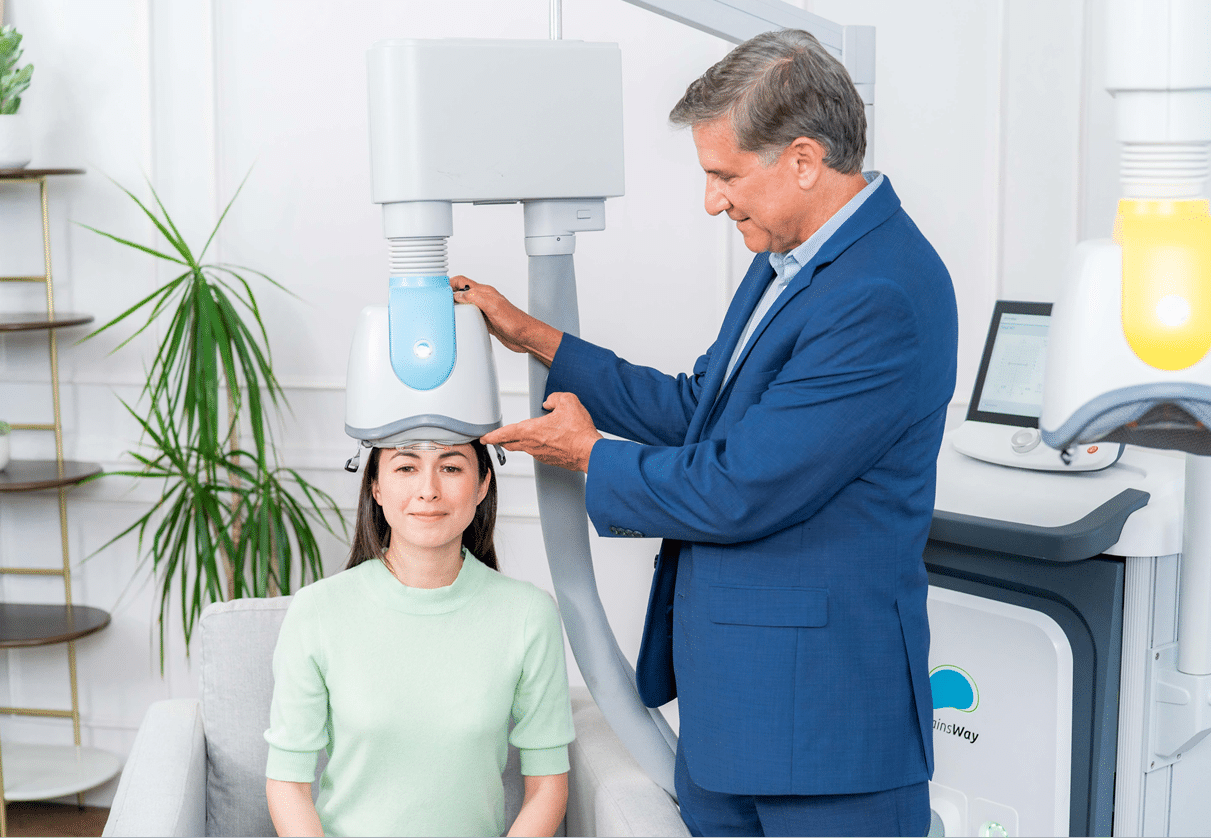
Non-invasive, safe, and medication-free, Deep TMS reactivates key brain regions responsible for mood and motivation. Thanks to BrainsWay’s exclusive H-Coil technology, it reaches deeper and broader brain areas than standard rTMS, offering the potential for faster and longer-lasting results.
Available only in St-Hubert for now.
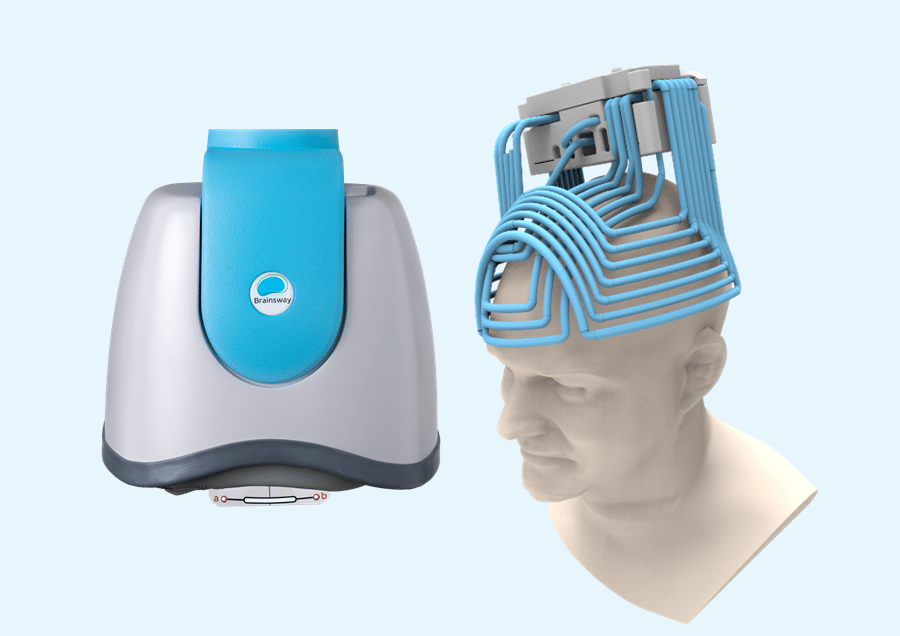
Standard rTMS uses a figure-8 coil to stimulate the superficial areas of the brain, which can be effective in certain cases of depression.
Deep TMS, however, uses a patented H-Coil helmet, allowing it to reach deeper and wider brain regions for faster, longer-lasting results, especially in cases of treatment-resistant major depression.
When antidepressants are no longer effective, the brain can remain stuck in disrupted activity patterns: some regions are under-stimulated while others are overactive.
Deep TMS sends gentle magnetic pulses that generate electrical activity capable of:
- Reactivating the brain’s reward circuits (the so-called “happiness zones”)
- Inhibiting anxiety and rumination networks
- Gradually rebalancing overall brain function
This process, performed without medication, anesthesia, or pain, promotes long-term improvement in mood and cognitive abilities.
While most clinics and hospitals offer treatment over 4 to 6 weeks, we have chosen to adopt an intensive protocol inspired by Stanford University research.
In just 5 days, you receive 50 sessions (10 per day), each lasting about 3 minutes. This approach, validated by clinical studies, has shown remission rates of up to 90% while minimizing disruptions to daily life.
The result? Our clients experience significant improvement more quickly, with fewer interruptions to their daily routine.
- *5-day intensive protocol – scientifically validated, faster results in a safe environment
- Exclusive Deep TMS technology – we are the only private clinic in Quebec offering it
- Medical referral included, at no extra cost (virtual appointment with our affiliated physician)
- Private rest room with TV, dining area, Wi-Fi, and ergonomic chair
- Healthy lunch included
- Access to an outdoor terrace for relaxation between sessions
- Welcome gift for every client
- Exclusive virtual training with our psychiatrist, to watch in your rest room between sessions and optimize lifestyle habits
*Please note: Accommodation is not included. If you are coming from outside the area, we recommend booking a hotel near our St-Hubert clinic.
We invite you to fill out the form to join our waiting list. As soon as the cohort is launched, you will be contacted.
This registration does not commit you to anything, but it will allow us to review your application and include you in the selection process.
To maximize benefits, Neuroperforma offers an intensive program:
- 50 sessions over 5 days (Monday to Friday)
- 10 short stimulations per day, each lasting 3 minutes
You remain at the clinic for the day, but we have planned everything to make your time enjoyable:
- Private relaxation room (TV, Netflix, dining area)
- Outdoor terrace and reading nook
- Chair for your companion if you come with someone
- Lunch included and welcome gift
Is accommodation included? *Please note that accommodation is not included. If you are coming from outside the area, we recommend booking a hotel near our St-Hubert clinic.
Initial fees
- File opening: $25
- Psychological assessment: $550
(60-minute consultation with a specialized psychologist to assess symptoms, history, and determine treatment eligibility)
- Psychiatric assessment: Free (covered by RAMQ)
(45-minute consultation with a psychiatrist – full review of medical history and medication adjustments if needed)
- Medical referral: Free
Treatment preparation
- Motor threshold setting: Included (approx. 15 minutes)
(The first treatment is slightly longer to adjust the motor threshold – necessary to precisely calibrate stimulation intensity to your physiology)
Intensive rTMS treatment
- 50 stimulation sessions: $175/session
Total: $8,750
Post-treatment follow-ups
- Follow-up #1: $250 (psychologist – Monday following treatment)
- Follow-up #2: $250 (psychologist – the following week)
This protocol is intended for adults with treatment-resistant major depression. f you have tried several antidepressants without satisfactory results, or if side effects have become too burdensome, rTMS could be a new solution.
Important: Certain contraindications — cranial metal implants, history of seizures, or specific neurological conditions — require personalized evaluation.
Yes. To save you the hassle, we provide a free medical referral.
Accommodation is not included in our services. If you are coming from outside the area, we recommend booking a hotel near our St-Hubert clinic to make your stay more comfortable.
Yes, at the moment, the treatment is offered exclusively at our St-Hubert clinic:
7750 Boul. Cousineau
Suite 403, Saint-Hubert, Quebec
J3Z 0C8
Coverage depends on your plan. Some insurers partially reimburse psychological consultations included in the protocol or offer a global mental health allowance you can apply toward this treatment. We recommend checking directly with your insurer for the exact terms of your coverage.
Yes, in many cases veterans can receive coverage for rTMS (Deep TMS) through Veterans Affairs Canada. If you are a veteran, we can help guide you through the process and provide the necessary documents to facilitate approval of your treatment.

